Abstract
Acute myeloid leukemia (AML) relapse post-allogeneic transplant (HSCT) has a dismal prognosis with a median survival of just 4.5 months. Conventional chemotherapy has limited efficacy in treating relapsed AML and is also poorly tolerated by allogeneic HSCT recipients. Similarly, the adoptive transfer of unmanipulated donor lymphocytes (DLIs) to provide a graft versus leukemia (GvL) effect has demonstrated clinical benefit in only a minority (~10%) of patients but frequently (>50%) induces debilitating graft versus host disease (GVHD). Thus, the goal of this project was to enhance both the safety and efficacy associated with adoptively-transferred donor-derived T cells by selectively enriching and infusing leukemia-directed populations that lack alloreactive potential.
To date we have generated 18 clinical grade T cell lines by stimulating donor PBMCs with dendritic cells loaded with overlapping peptide libraries spanning the leukemia-associated antigens (LAA) - WT1, PRAME, NY-ESO1 and Survivin. The lines were polyclonal, comprising both CD4+ (26.7±3.5%) and CD8+ (44±2.9%) cells and expressed memory markers CD45RO+/CD62L-/CCR7- (29.3±4%) associated with long term in vivo persistence. The lines were specific for the targeted antigens WT1, PRAME, NY-ESO1 and Survivin by IFNg ELIspot, and did not exhibit any activity against non-malignant patient-derived cells (3±3.5%, E:T 20:1) - a study release criterion.
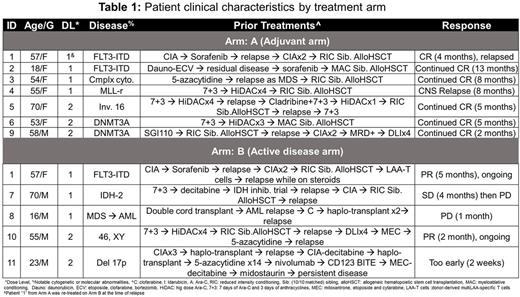
To test the safety and clinical effects of these donor-derived multiLAA T cells, we developed a phase I clinical trial - ADSPAM (IND #16700) to infuse escalating doses of cells (ranging from 0.5 to 2x107 cells/m2) asadjuvant therapy for those with adverse prognosis AML (Arm A) or to treat active, relapsed disease (Arm B). To date, 12 patients (prior histories summarized in Table 1) have been infused with multiLAA T cells (7 on Arm A and 5 on Arm B). Of note, pt1, was treated on both Arms A and B and except for this patient, who had a transient elevation of liver enzymes (AST and ALT) 2 weeks after the infusion of T cells (on Arm A at 0.5 x 107 cells/m2), there were no other infusion-related adverse events.
Of the 7 patients who were infused on the adjuvant arm (Arm A), 2 (pts 1 and 4) developed relapsed disease. Pt4 developed brain parenchymal and leptomeningeal relapse 8 months post-multiLAA T cells and 10 months post-allogeneic HSCT, while pt1 relapsed in the bone 4 months post-multiLAA T cells (9 months post-allogeneic-HSCT) while receiving prednisone. Given the potential impact of the steroids on circulating T cells this patient was subsequently re-enrolled and treated on our active disease arm.
Among 5 patients infused with multiLAA T cells to treat relapsed disease, 1 is still too early, 2 developed disease progression (months 1 and 3 post-multiLAA T cells, respectively) and 2 (pts 1 and 10) had clinical responses. Pt10, with a history of chemotherapy- and DLI-refractory AML and 30-40% bone marrow blasts at the time of infusion had a 50% reduction in disease burden within 1 month of infusion and remains in active follow-up (currently month 2 post-infusion) with additional multiLAA infusions planned. Pt1, who developed an extramedullary AML relapse at multiple sites within the axial skeleton, had a complete resolution of all disease-related symptoms (back and hip pain and limited range of motion at the shoulder) and a near complete resolution of all bony lesions, based on imaging studies, within 1 month of infusion. Currently, this patient remains disease-free 5 months post-reinfusion on Arm B and 1.5 years from transplant. Interestingly, in this patient whose blasts expressed WT1, we detected a marked and sustained increase in the circulating frequency of WT1-directed T cells in both peripheral blood and marrow that were confirmed of multiLAA origin based on TCR deep sequencing. In summary, infusion of donor multi-LAA-specific T cells to patients with AML post allogeneic HSCT is safe and associated with clinical benefit in individuals with highly treatment-resistant leukemias.
Lulla: Leukemia Texas Research grant: Research Funding; Junior Faculty sees funding-Baylor College of Medicine: Research Funding; ASH Scholar Award: Research Funding; Lymphoma SPORE: Research Funding; ASBMT Young Investigator Award: Research Funding; Leukemia Texas: Membership on an entity's Board of Directors or advisory committees. Tzannou: ViraCyte LLC: Consultancy. Heslop: Viracyte: Equity Ownership; Cell Medica: Patents & Royalties: EBV specific T cells, Research Funding; Marker Therapeutics: Equity Ownership; Celgene: Research Funding. Vera: ViraCyte LLC: Equity Ownership; Wilson Wolf Manufacturing: Consultancy; Marker Therapeutics: Equity Ownership. Leen: ViraCyte LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.